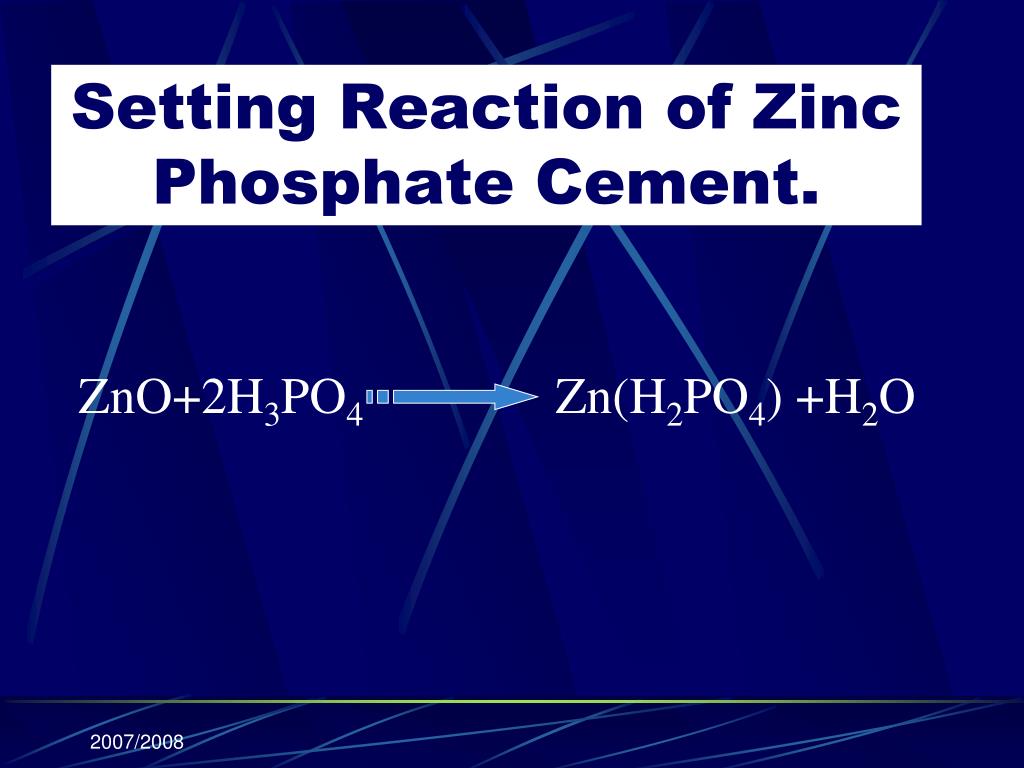
Zinc Acetate And Sodium Phosphate Reaction Formula . Both the hydrate and the anhydrous forms. there are three main steps for writing the net ionic equation for na3po4. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). the solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid. two reactants znc2h3o2 and na3po4 react to form an aqueous product and a white precipitate. this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. write the net ionic equation for any reaction that occurs.
from www.slideserve.com
the solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid. a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). write the net ionic equation for any reaction that occurs. there are three main steps for writing the net ionic equation for na3po4. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. two reactants znc2h3o2 and na3po4 react to form an aqueous product and a white precipitate. Both the hydrate and the anhydrous forms. An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii).
PPT DENTAL CEMENTS PowerPoint Presentation, free download ID2825532
Zinc Acetate And Sodium Phosphate Reaction Formula write the net ionic equation for any reaction that occurs. Both the hydrate and the anhydrous forms. An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). the solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid. this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. there are three main steps for writing the net ionic equation for na3po4. write the net ionic equation for any reaction that occurs. a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. two reactants znc2h3o2 and na3po4 react to form an aqueous product and a white precipitate.
From chemwiki.ucdavis.edu
Section 10.2 Phosphorylation reactions kinase enzymes Chemwiki Zinc Acetate And Sodium Phosphate Reaction Formula two reactants znc2h3o2 and na3po4 react to form an aqueous product and a white precipitate. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). . Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.dreamstime.com
Zinc Acetate is a Molecular Chemical Formula. Zinc Infographics. Vector Zinc Acetate And Sodium Phosphate Reaction Formula two reactants znc2h3o2 and na3po4 react to form an aqueous product and a white precipitate. there are three main steps for writing the net ionic equation for na3po4. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. the. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.numerade.com
SOLVED Write the balanced molecular equation, including phases, for Zinc Acetate And Sodium Phosphate Reaction Formula zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. two reactants znc2h3o2 and na3po4 react to form an aqueous product and a. Zinc Acetate And Sodium Phosphate Reaction Formula.
From campbellderne1998.blogspot.com
Write The Balanced Chemical Equation For Each Of These Reactions Zinc Acetate And Sodium Phosphate Reaction Formula Both the hydrate and the anhydrous forms. there are three main steps for writing the net ionic equation for na3po4. An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). write the net ionic equation for any reaction that occurs. zinc acetate is a salt with the formula zn(ch 3 co 2) 2,. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.chegg.com
Solved Write the net ionic equation for the reaction between Zinc Acetate And Sodium Phosphate Reaction Formula write the net ionic equation for any reaction that occurs. Both the hydrate and the anhydrous forms. An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. the solid sodium reacts with liquid water to produce. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.animalia-life.club
Lewis Dot Structure Sodium Zinc Acetate And Sodium Phosphate Reaction Formula two reactants znc2h3o2 and na3po4 react to form an aqueous product and a white precipitate. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. write the net ionic equation for any reaction that occurs. the solid sodium reacts. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.slideserve.com
PPT Reactions Involving Ions Molecular vs. Ionic Equations Zinc Acetate And Sodium Phosphate Reaction Formula write the net ionic equation for any reaction that occurs. the solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid. a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. zinc acetate is a salt with the formula zn(ch. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.researchgate.net
Schematic illustration of the zinc phosphate mechanism with passive Zinc Acetate And Sodium Phosphate Reaction Formula An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). Both the hydrate and the anhydrous forms. two reactants znc2h3o2 and na3po4 react to form an aqueous product and a white precipitate. this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). write the net ionic equation for any. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.youtube.com
Equation for Na3PO4 + H2O (Sodium phosphate + Water) YouTube Zinc Acetate And Sodium Phosphate Reaction Formula zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). there are three main steps for writing the net ionic equation for na3po4. a precipitation. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.youtube.com
What is the formula of zinc acetate? YouTube Zinc Acetate And Sodium Phosphate Reaction Formula An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). there are three main steps for writing the net ionic equation for na3po4. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. Both the hydrate and. Zinc Acetate And Sodium Phosphate Reaction Formula.
From paul-chapter.blogspot.com
Balanced Equation For Zinc Acetate And Sodium Phosphate 39+ Pages Zinc Acetate And Sodium Phosphate Reaction Formula a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. there are three main steps for writing the net ionic equation for na3po4. Both the hydrate and the anhydrous forms. the solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid.. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.researchgate.net
(a) The mechanism for the reaction of zinc acetate and DEA in Ethanol Zinc Acetate And Sodium Phosphate Reaction Formula this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). there are three main steps for writing the net ionic equation for na3po4. write the net ionic equation for any reaction that occurs. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.slideserve.com
PPT Chemistry in Review The Ions Chemical Formulas Chemical Zinc Acetate And Sodium Phosphate Reaction Formula the solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. there are three main steps for writing the net ionic. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.pw.live
Zinc Phosphate Formula, Properties And Uses Zinc Acetate And Sodium Phosphate Reaction Formula the solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid. a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. there are three main steps for writing the net ionic equation for na3po4. write the net ionic equation for. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.slideserve.com
PPT Chapter 4 The Major Classes of Chemical Reactions PowerPoint Zinc Acetate And Sodium Phosphate Reaction Formula this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). there are. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.researchgate.net
Schematic diagrams of mechanism of inhibition of zinc phosphate Zinc Acetate And Sodium Phosphate Reaction Formula a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. write the net ionic equation for any reaction that occurs. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. this video. Zinc Acetate And Sodium Phosphate Reaction Formula.
From achs-prod.acs.org
Chemical Equilibrium of Zinc Acetate Complexes in Ethanol Solution. A Zinc Acetate And Sodium Phosphate Reaction Formula write the net ionic equation for any reaction that occurs. An aqueous solution of strontium hydroxide is added to an aqueous solution of iron(ii). this video discusses the reaction between (zinc acetate) zn (c2h3o2)2 + na3po4 (sodium phosphate). there are three main steps for writing the net ionic equation for na3po4. two reactants znc2h3o2 and na3po4. Zinc Acetate And Sodium Phosphate Reaction Formula.
From www.numerade.com
SOLVED Aqueous solutions of zinc bromide and sodium phosphate combine Zinc Acetate And Sodium Phosphate Reaction Formula a precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Both the hydrate and the anhydrous forms. zinc acetate is a salt with the formula zn(ch 3 co 2) 2, which commonly occurs as the dihydrate zn(ch 3 co 2) 2 ·2h 2 o. two reactants znc2h3o2 and na3po4 react. Zinc Acetate And Sodium Phosphate Reaction Formula.